Rapid Test
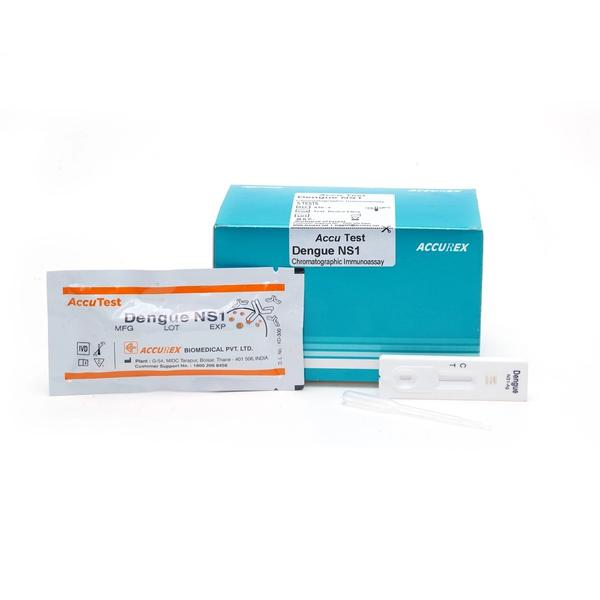
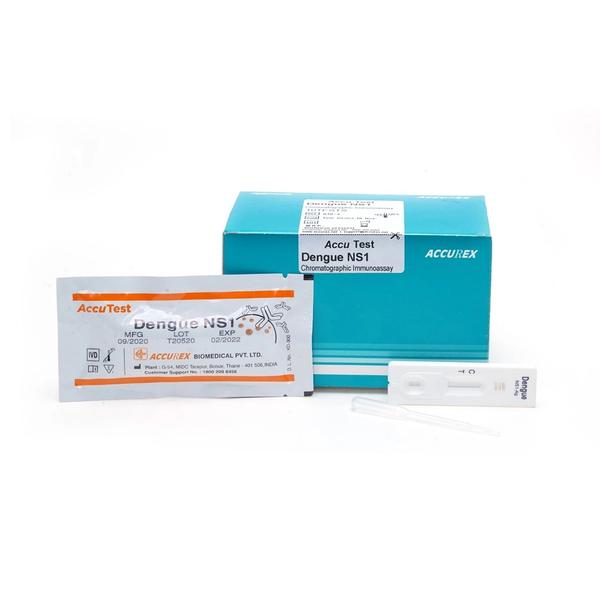
Buy Dengue NS1 Antigen test (Pack of 10 test) from Accurex. This NS1 Antigen Rapid test contains: A Test device in aluminum pouch with a desiccant, Disposable dropper & Pack Insert. Dengue NS1 antigen test utilizes the human serum / plasma followed by solid-phase immuno-chromatographic technology for the qualitative detection of dengue virus NS1 antigen. The membrane strip of the kit contains pre coated anti-dengue NS1 monoclonal antibody on the test band region (T), & goat anti-mouse IgG is pre-coated on the control band region (C).
EXPLANATION OF THE TEST
Dengue NS1 antigen test utilizes the human serum / plasma followed by solid-phase immuno-chromatographic technology for the qualitative detection of dengue virus NS1 antigen. The membrane strip of the kit contains pre-coated anti-dengue NS1 monoclonal antibody on the test band region (T), and goat anti-mouse IgG is pre-coated on the control band region (C). During testing, if the sample containing dengue NS1 Ag, the complex of the antibody-dengueNS1 Ag-gold conjugate moves laterally on the membrane by capillary action. In this case, the colored band will appear on the membrane in test line (T). Control line (C) should always appear if the test procedure is performed properly
PRECAUTIONS
1. For in-vitro diagnostic use only. Do not re-use the test device. 2. The instruction must be followed exactly to get accurate results. 3. Anyone performing an assay with this product must be trained in its use and must be experienced in laboratory procedures. 4. Do not eat or smoke while handling specimens.
5. Wear protective gloves while handling specimens. Wash hands thoroughly afterwards.
6. Avoid splashing or aerosol formation.
7. Clean up spills thoroughly using an appropriate disinfectant. 8. Decontaminate and dispose of all specimens, reaction waste, in a biohazard container.
9. Do not mix and interchange different specimen.
10. The presence of humidity may decrease the stability of the tests. Thus, carry out the test immediately after removing the device from the foil pouch.
11. Do not use it beyond the expiration date.
SPECIMEN COLLECTION AND STORAGE
Direction for Use
1. Place all specimens, test device and solution. Allow them to room temperature prior to testing (15~30 min.)
2. Please perform the test immediately after removing the device from the foil pouch.
3. With a disposable dropper, load 2-3 drops (50 to 75µl) of specimen into the sample well (S) in the test device.
4. Interpret the test results between 15~20 minutes. Do not read the results after 20 minutes.
Other Info
STORAGE & EXPIRATION
1) Dengue NS1 Antigen test should be stored between 4~30 ºC
2) Expiration date of this kit is 24 months after its manufacture date.
LIMITATIONS OF THE TEST
Dengue NS1 antigen test is designed for primary screening test of
dengue virus NS1 antigen. Although this can provide fast and easy
way to get a result, the testing do not completely exclude the possibility
of false positive or negative result caused by various factor. So, refer
to the result of the kit, please make a final decision with clinical
manifestation, other test results and doctor’s view collectively.
Send Message