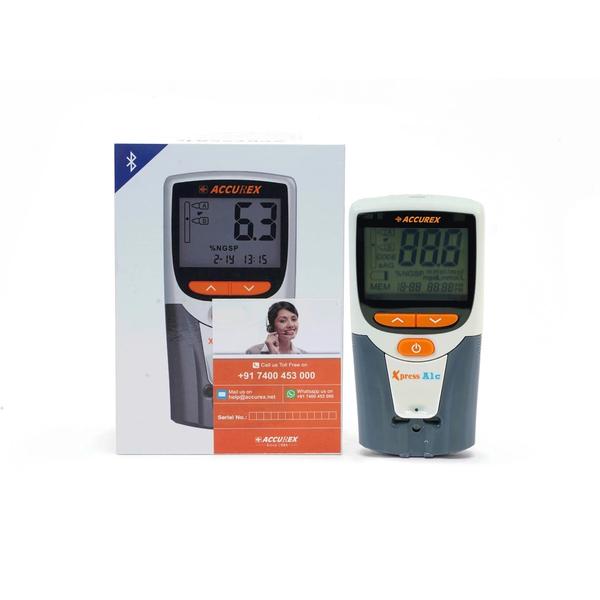
Accurex Xpress A1c Glycohemoglobin Analysis System Kit . The Kit contains HbA1c Meter, 1 Sampler, 1 box of 25 test strips, Buffer A, Buffer B. Glycohemoglobin Analyzer It is used for testing glycohemoglobin concentration as an aid to monitor the risk of developing diabetes & control the status of diabetes. Buy Xpress A1c Meter Kit Online from Accurex Point of Care (POC ) Range – The HbA1c analyzer is specifically designed to be used with the corresponding test strip. HbA1c analyzer Hemoglobin A1C is a common blood test that measures blood glucose levels over the past two to three months. •Glycohemoglobin Analyzer •Handheld HbA1c Meter •Battery Operated •No Pipetting, No Lysing •Lab like Accuracy •Battery Operated •Truly Portable •Voice prompt for •User Guidance • Accurate result with cv <3% •Lab like accuracy with 95% •No interference of HB Variant •No pipetting •No lysing •Reagent: Suitable to store at room temperature (upto 30º C) The Power of HbA1c in your Hand esting Principle: Boronate Affinity Chromatography Testing Parameter: Glycohemoglobin (HbA1c) Measuring Range: 4.0% – 14.0% Precision: CV <3% (HbA1c: 4.0% – 6.5%) Blood Sample: Finger Prick or Venous Blood ( EDTA Anticoagulant) Blood Volume: About 3ul Testing Time: About 5 minutes Data Unit: Set in Advance the Data Unit • NGSP%; IFCC mmol/mol; eAG* mmol/l Voice Prompt: Voice Prompt to Guide the user Data Storage: 1000 Testing Data Data Port: Mini USB data interface can be connected with HIS/LIS; System Thermal Printer Power Required: AAA Battery x 4 Analyzer Dimension: 61.6 x 122.9 x 24.5 mm Screen Size: 47 x 32 mm” Safety Information LIMITATION AND WARNING: 1. This product is used for In Vitro Diagnostics only, not for in vivo use. 2. This product should not be used past the expiration date. 3. If there is evidence of microbial contamination or browning, discard the vial. 4. This product is not intended to be used as an HbA1c standard. 5. Preservatives contained in the control, rinse with water if splash into the mouth or eyes, and seek medical help as soon as possible. 6. Potential biological risk. Direction for Use This product should be run in accordance with instructions accompanying BioHermes Glycohemoglobin Test Kit and Glycohemoglobin Analyzer. (1) Dissolution: Open the cap of the vial, and then add 2 drops of Buffer A. Close the cap, and slightly shake the vial until the powder dissolved completely. And please shake it well before using it. (2) Testing in accordance with instructions accompanying BioHermes Glycohemoglobin Test Kit and Glycohemoglobin Analyzer. Please refer to the B Other Info Storage: 1. This product will be stable for 24 months when stored tightly capped at 2-8℃. 2. After dissolved, the product can be stable for 8 hours at 10-30℃, or 5 days at 2-8℃. Do not freeze it. COMPOSITION: This product is prepared from human blood and contains normal hemoglobin, preservatives and stabilizers. The control is provided in lyophilized powder form for increased stability. The kit contains two levels of control, level 1 is with yellow cap, and level 2 is with green cap.
Send Message