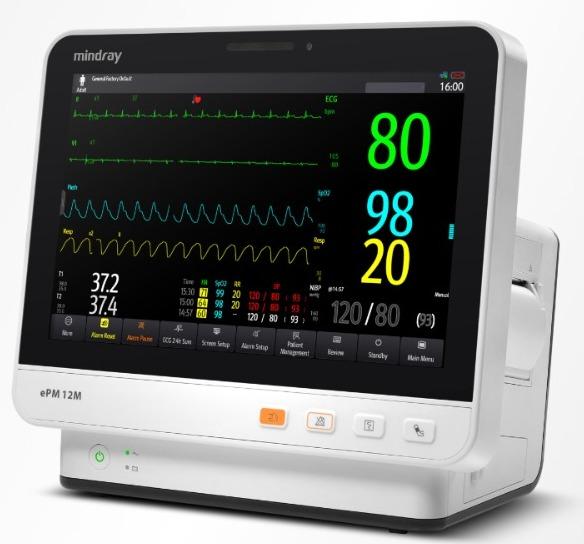
With state-of-the-art screen technology, BeneVision N Series patient monitors deliver clear, multi-color, wide-format displays for users to capture and review information at a glance. With multi-touch operation, users can control the monitor and review patient data quickly and easily. BeneVision N Series provides the world's best monitoring technologies for you, with new applications being developed. Perfect hemodynamic monitoring solution, not only provides a variety of measurement methods, ICG, PiCCO, ScvO2, C.O., but also provides comprehensive analysis tool – HemoSight™. HemoSight™ focuses on hemodynamic treatment process, and provides appropriate tools for each stage. Advanced modular regional tissue oxygenation measurement -- INVOS rSO2. Volumetric capnography & metabolic measurement help to evaluate the adequacy of ventilation and determine the ventilator setting effectively, and improve the safety of weaning and extubation. Powerful Clinical Assistive Applications (CAAs) to support efficient decision making when time is critical. Each CAA focuses on major clinical workflow challenges that individual departments face. Solutions optimized for each point of care At every point of care, such as ICU, CCU, NICU, OR, PACU, ER, BeneVision N Series patient monitors always provide a suitable solution to meet your clinical needs for monitoring your patient's status anywhere, anytime, even on your way through mobile devices. With excellent transport solution, BeneVision N Series patient monitor brings a smooth workflow and safe data management through the entire care process. BeneVision takes less time to operate, and helps you understand the patient's variables quickly. With HL7,BeneVision N Series patient monitors can directly connect to the hospital clinical network.BeneVision N12 is capable of monitoring multiple parameters simultaneously both at bedside and during transport. IT structure designed for seamless connectivity Mindray patient monitoring system incorporates extensive network adaptability to integrate with the hospital's current network infrastructure, ensuring that critical data is on hand for clinical decision making - and is integrated with the patient record. Mindray's central station and eGateway further enhances the connectivity of BeneVision to your clinical world. Bedside devices data and other clinical systems data are shared to support your diagnosis and clinical decisions.
Send Message