Rapid Test
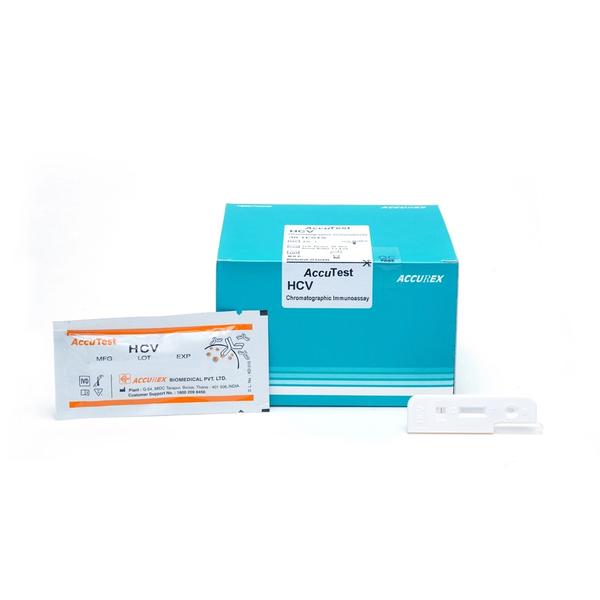
Buy AccuTest HCV Rapid Card Test (30 Tests) Online from Accurex. HCV means Hepatitis C Virus which is a is a small, enveloped, positive-sense, single-stranded RNA virus. Hepatitis C symptoms are fever, fatigue, decreased appetite, nausea, vomiting, abdominal pain, dark urine, pale feces, joint pain and jaundice. HCV test is a rapid Chromatographic Immunoassay work on principle of the double antigen sandwich technique, Qualitative detection of antibodies generated against proteins that are encoded by conserved sequence of core NS3, NS4, NS5 parts of HCV genome in human whole blood/serum or plasma.
AccuTest HCV Test is a rapid Chromatographic Immunoassay for the Qualitative detection of antibodies generated against proteins
that are encoded by conserved sequence of core NS3, NS4, NS5 parts of HCV genome in human whole blood/serum or plasma.
Hepatitis C Virus (HCV) is a small, enveloped, positive-sense, single-stranded RNA virus. Antibody to HCV is found in over 80% of
patients with well documented non-A, non-B hepatitis. Conventional methods fail to isolate the virus in cell culture or visualize it by
electron microscope. Cloning the viral genome has made it possible to develop serologic assays that use recombinant antigen,
multiple antigens using recombinant protein and/or synthetic peptides have been added in new serologic tests to avoid nonspecific
cross-reactivity to increase the sensitivity of HCV antibody tests.
Storage and Stability:
AccuTest HCV should be stored at 4-30C. However, the card may be stored at room temperature not exceeding 30C in the original
sealed pouch.
Test Procedure:
1. Allow the test, specimen and or control to room temperature prior to the testing
2. Remove one test card from the pouch and place it on a clean flat surface
3. Using the dropper provided one drop of serum/plasma or W. blood sample (30ul) then two drops of buffer (40ul) immediately into the sample well. Avoid overflowing
4. Read results within 15 minutes. Strong positive reaction may visible within 5 minutes. Do not read results after 15 minutes.
5. If negative or quest
Principle:
AccuTest HCV Rapid Hepatitis C Virus Test (Serum/Plasma/Whole Blood) is a lateral flow chromatographic immunoassay based on
the principle of the double antigen-sandwich technique. The membrane is coated with recombinant HCV antigen on the test line
region of the device. During testing, the serum/plasma or W.Blood specimen reacts with HCV antigen coated particles. The mixture
migrates upward on the membrane chromatographically by capillary action to react with recombinant HCV antigen or membrane
and generate a colored line. Presence of this colored line indicates a positive result, while its absence indicates a negative result. To
serve as a procedural control, a colored line will always appear at the control line region, indicating that the correct volume of
specimen has been added and membrane wicking has occurred.
Storage and Stability:
AccuTest HCV should be stored at 4-30C. However, the card may be stored at room temperature not exceeding 30C in the original
sealed pouch.
Test Procedure:
1. Allow the test, specimen and or control to room temperature prior to the testing
2. Remove one test card from the pouch and place it on a clean flat surface
3. Using the dropper provided one drop of serum/plasma or W. blood sample (30ul) then two drops of buffer (40ul)
immediately into the sample well. Avoid overflowing
4. Read results within 15 minutes. Strong positive reaction may visible within 5 minutes. Do not read results after 15 minutes.
5. If negative or questionable results are obtained, the HCV infection is suspected, the test should be repeated ona fresh serum specimen.
Safety Information
•Positive: If a distinct purple line is formed at the test one marked ‘T’ (Test Line) and the Control Zone marked ‘C’ (Control
Line) the test result is positive, indicating the sample contains Hepatitis C antibody. The interpretation of test results (+ve
for hepatitis) remains unchanged even if there is a difference in intensity of color in positive line and control line as is many
a time found.
•Negative: If a distinct purple line is formed only at the control zone marked ‘C’ (control line) the test result is negative
• Invalid: A total absence of color in both © and (T) regions or no colored band appears on the control © region is an
indication of procedure error and/or the test reagent has deteriorated. Repeat with a new test kit.
Direction for Use
Test Procedure
1. Allow the test, specimen and or control to room temperature prior to the testing
2. Remove one test card from the pouch and place it on a clean flat surface
3. Using the dropper provided one drop of serum/plasma or W. blood sample (30ul) then two drops of buffer (40ul)
immediately into the sample well. Avoid overflowing
4. Read results within 15 minutes. Strong positive reaction may visible within 5 minutes. Do not read results after 15
minutes.
5. If negative or questionable results are obtained, the HCV infection is suspected, the test should be repeated ona fresh
serum specimen.
Other Info
Storage and Stability
AccuTest HCV should be stored at 4-30C. However, the card may be stored at room temperature not exceeding 30C in the original
sealed pouch.
Send Message