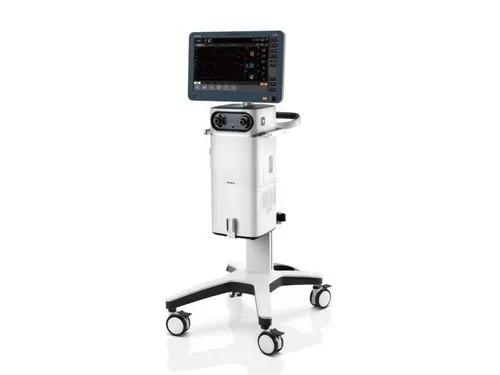
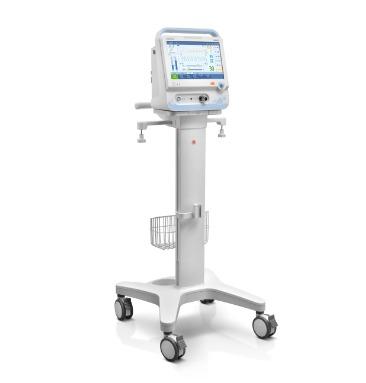
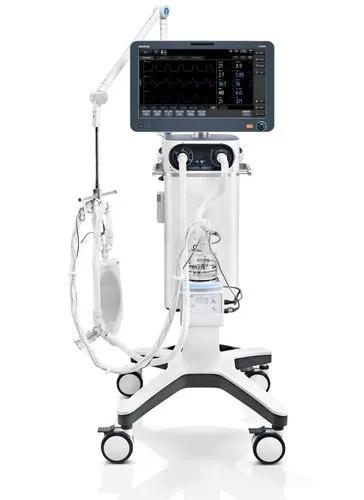
A high-end ventilator featured with 1080P HD wide screen, combines an intuitive customized UI with powerful assistive tools and modules. Operate with Ease In the modern busy clinical environment, ease of use is a fundamental requirement for all medical devices. With this in mind, the new SV800/SV600 ventilators enable clinicians to set and deliver ventilation therapies quickly and easily via the intelligent ergonomic design and flat user interface. Intelligent Decision Making Ventilation modes and decision-supporting tools like SBT are developed on the basis of clinical needs and professional guidelines to help free up medical personnel’s time on device operation and focus more on their patients. p68-s4 Extensive ventilation therapy and decision support tools Smart ventilation solution: AMV TM + IntelliCycle TM Emergency solution: CPRV TM Sequential treatment regimen: Non-invasive ventilation & high flow oxygen therapy Dual channel auxiliary pressure measurements Lung protection kit Expandable Capabilities Securing your devices future is reliant on being able to expand your devices capabilities by interacting or integrating new features and technologies. The SV800/SV600 ventilators allow just this. Thanks to latest software and platform it is utilizing, your device is ready to embrace new technological advancement with ease. p68-s0-0 Integrated neonatal module with tidal volume setting down to 2ml Plug & Play modules , including SpO₂, mainstream or sidestream CO₂ Backup air supply in case of central air supply failure and during intra-hospital transport Seamlessly connected to the hospitals clinical information system via the Mindray BeneLink and eGateway modules, or directly via the HL7 p68-s5 Brand - Mindray Model Name/Number SV800 Patient Age Group Adult, Pediatrics & Neonatal Tidal Volume For adult : 100 to 4000 ml, For Pediatric 20 to 300 ml, For neonatal : 2 to 100 ml Ventilation Mode V-A/C, P-A/C, V-SIMV,P-SIMV, Duolevel, CPAP, PSV, APRV, PRVC, NIV, PRVC-SIMV, AMV, CPRV, PSV-S/T, nCPAP, VS Respiratory Rate For adult/pediactrics : 1 to 100/min,for neonate :-1 to 150/min Additional Details Weight 45 kg ventilator + 16.3 kg trolley Plateu (%) OFF, 5% to 60% Peak airway pressure 0 to 100 cm H2O SIMV Rate ,Cycle/min 1 to 60 rpm Mounting type Trolley I:E Ratio 1:10 to 4:1 Touch screen 18.5 inch color active matrix TFT touch screen RSBI 0-9999/1/(L-min) Features Customizing the UI Graphic guidelines Single level menu design Dual channel auxiliary pressure monitore
Send Message