Fluorescence Immunoassay Analyzer
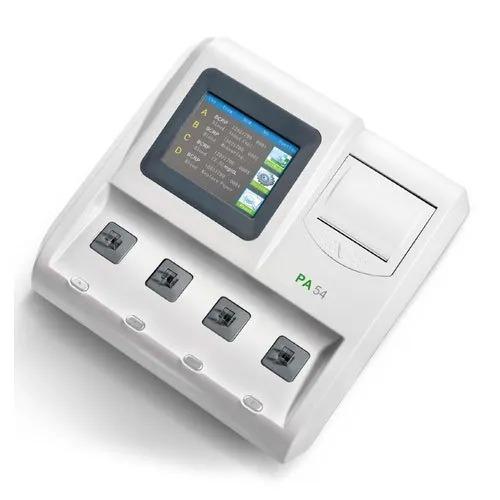
The FineCare FIA Meter II plus SE is a top-class fluorescence immunoassay analyzer with a built-in thermal printer, Wifi, internet connectivity, large LCD display for testing common clinical diseases.
Description
The FineCare FIA Meter II plus is a top-class fluorescence immunoassay analyzer with a built-in thermal printer, Wifi, and internet connectivity, large LCD display for testing common clinical diseases.
Technical Specifications
Analyses common clinical diseases such as Diabetic & Renal injury Markers, Thyroid function, Fertility, Cardiac Markers, Inflammation Markers, Tumour Markers
Built-in Thermal Printer
Real-time auto printing
Support for external printer
Dimension :- 239mm x 278mm x 148mm
Weight : 3.3kg
Large Display
8” LCD Capacitive touch screen Intuitive user interface
Specially designed sampler for a small volume of the specimen.
Test Reagent
Suitable for whole blood, serum, plasma, and urine
Room temperature storage
Get results in 3 -15 minutes
Test Device Tray
Multiple testing modes available
Unique quick mode for a large number of samples
Keywords
large number
fluorescence
small volume
Cardiac Markers
Thyroid function
large LCD display
Inflammation Markers
Renal injury Markers
internet connectivity
common clinical diseases
LCD Capacitive touch screen Intuitive user
Built-in Thermal Printer
Immunoassay Analyzer, Finecare Analyzer, TSH, HbA1c, Point of care , immunoassay, Clia
Analyses common clinical diseases such as Diabetic & Renal injury Markers, Thyroid function, Fertility, Cardiac Markers, Inflammation Markers, Tumour Markers
Send Message