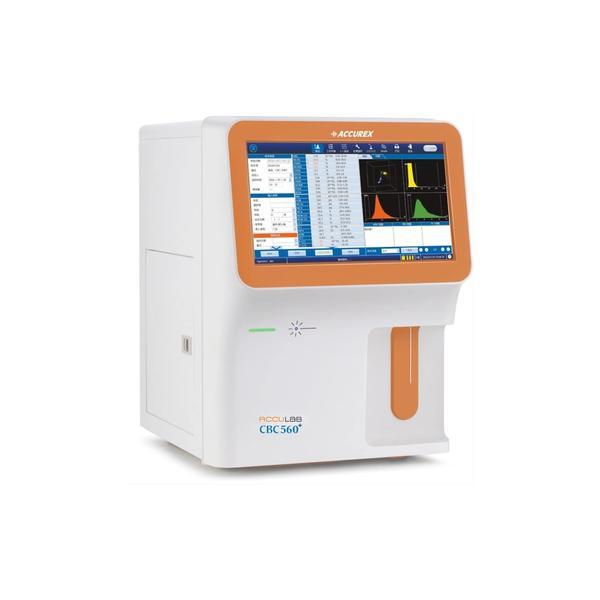
Buy the Best 5 Parts Hematology Analyzer – ACCULAB CBC 560+. This Hematology Analyser Machine is an advanced and automatic hematology analyzer, comprising 5 parts, 29 parameters, and 3 histograms assay, offers throughput of 60 tests/hour. Features: Tri-angle laser scatter, Flow cytometry method, Impedance method for RBC and PLT counting and Cyanide-free method for HGB test along with 3D holographic scattergram to display the accurate 5 part differentiation of WBC. Equipped with a 14 inch touch screen and large storage capacity for 100,000 results, this hematology analyzer has interface of 4 USB ports, 1 LAN port Bi-direction LIS, support HL 7 protocol, Internal RFID reader. With CBC mode, CBC+DIFF mode, Venous whole blood, Capillary whole blood and Prediluted test mode, this hematology analyzer is an ideal unit for white blood cell counts, complete blood Measuring principle Tri-angle laser scatter, Flow cytometry method, Impedance method for RBC and PLT counting, Cyanide-free method for HGB test Sample volumes CBC+DIFF mode: ≤ 20 μl CBC mode: ≤ 10 μl Throughput 60 tests/hour Assay Items 29 parameters, 3 histograms Display 14 inch touch screen Storage 100,000 sample results, including histograms, scattergrams and patient information Parameter WBC, RBC, HGB, HCT, MCV, MCH, MCHC, RDW-SD, RDW-CV, PLT, MPV, PCT,PDW, P-LCR, P-LCC, NEU%, LYM%, MON%, EOS%,BAS%,NEU#, LYM#, MON#, EOS#, BAS# 4 Research parameter: ALY%, ALY#, LIC%, LIC# Performance Item Linearity range Carry Over CV WBC 0-300 × 109/L ≤ 0.5% ≤ 2.0 % RBC 0-8.00 ×1012/L ≤ 0.5% ≤ 1.5 % HGB 0-250 g/L ≤ 0.5% ≤ 1.5 % PLT 0-3000 × 109/L ≤ 1.0% ≤ 4.0 % Interface 4 USB ports, 1 LAN port Bi-direction LIS, support HL 7 protocol, Internal RFID reader Power Consumption 400 VA Power Supply AC 220V±10%, 50/60Hz, 110V±10%, 60Hz Packaging dimension (W×D×H) 670 × 590 × 790 mm Gross Weight 53 kg Safety Information All the samples, controls, calibrators, reagents, waste and areas contacted with them are potentially biohazardous. Wear proper personal protective equipment (e.g. gloves, lab coat, etc.) and follow safe laboratory procedures when handling them in the laboratory.
Send Message