Clinical Chemistry
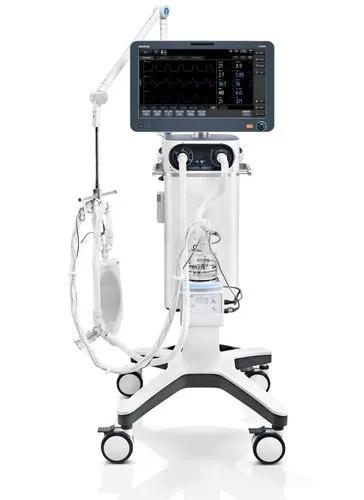
ADVIA 2400 Chemistry System The right balance of speed and efficiency
The ADVIA® 2400 Clinical Chemistry System manages the most demanding workloads and meets high turnaround-time (TAT) goals. Its menu includes methods for testing drugs-of-abuse, therapeutic drugs (TDM) and new assays such as cystatin C and CardioPhase® hsCRP. The ADVIA 2400 Clinical Chemistry System offers:
High throughput of up to 2400 tests/hour (1800 photometric and 600 ISE) to manage heavy workloads
Rapid, consistent, 2-second cycle time for faster tube speed and test throughput
User-defined automatic repeat, dilution, and reflex testing
Large onboard reagent capacity and optional concentrated reagents help to minimize intervention demands
Refrigerated onboard storage of controls and reagents for extended stability and increased productivity
Automation-ready design: no additional hardware required
Features & Benefits
The ADVIA 2400 Clinical Chemistry System meets today’s high turnaround-time goals with a throughput of up to 2400 tests/hour. In addition to assays for general and specialty chemistries, its menu includes assays for specific protein measurement, drugs-of-abuse testing (DAT), and therapeutic drug monitoring (TDM). Interesting new assays such as cystatin C, HbA1c with automated sample pretreatment, and CardioPhase® hsCRP can now be included in your routine testing.
The ADVIA 2400 Chemistry System pushes productivity levels while maintaining the reliability and stability that keep laboratories up and running. A rapid, 2-second probe cycle time maximizes tube speed and test throughput and combines with the system’s efficient automation-readiness to provide the turnaround time needed for high-volume laboratories
Superior Speed and Productivity
Throughput of up to 2400 tests/hour (1800 photometric and 600 ISE) to manage the heaviest workloads
Rapid, consistent, 2-second cycle time
Throughput of 200 tubes per hour, even when running three ISE tests and nine photometric tests per sample
Automatic Sample Retain technology, auto-repeat, auto-dilution, and auto-reflex testing
Automation-ready
Point-in-space aspiration enables connectivity to a track.
Redundant sample loading provides built-in backup and easy handling of non-routine samples.
Direct sampling from track prevents tubes being detained at an analyzer and reduces aliquoting.
Minimal Need for Operator Intervention
Minimal-maintenance ISEs
Large onboard reagent capacity and optional concentrated reagents for fewer interruptions
No-maintenance, 37°C incubation oil bath
Auto-calibration and auto-QC from refrigerated onboard calibrators and controls
Microvolume technology with 32,000 tests onboard and up to 60 days' onboard stability for improved walkaway time. Concentrated reagents for increased maximum capacity.
Remote diagnostics and Inter-Laboratory Quality Control (ILQC) options
Exceptional Reliability
Sample integrity check for icterus, hemolysis, and lipemia
Sample probe with automatic clot/clog detection, liquid-level sensing, short-sample detection, and crash protection
Advances in onboard reagent stability, calibration frequency, reduction in interferences, and expanded assay linearity
Send Message