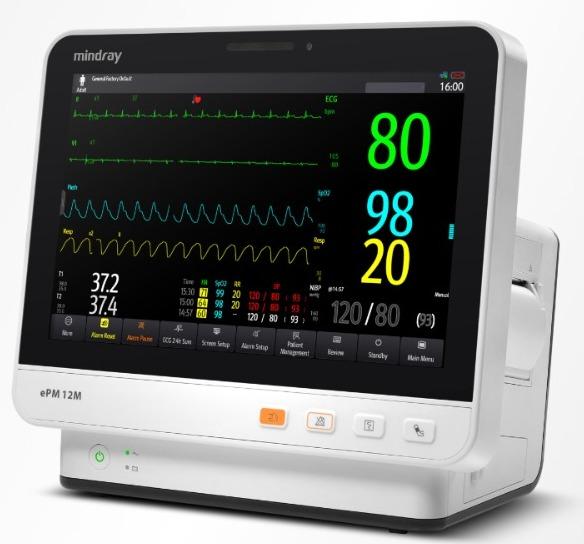
With cutting-edge LED technology, the HyLED 7 Series offers excellent luminous efficiency, heat-free light source, and incredible long service life, giving you total support in OR. Usage/Application Operation Theater Positioning Ceiling Mounted Model Name/Number HyLED 7 Series Brand Mindray Light Source Type LED Central illuminance at 1m distance 160,000 lux Light field diameter(at 1m distance) 180~300 mm Depth of illumination(L1+L2) 1,200 mm Correlated color temperature 4,350 K Color rendering index(Ra) 96 Color rendering index(R9) 97 Shadow dilution with tube 100% Shadow dilution with one mask 75% Shadow dilution with tube and one mask 70% Shadow dilution with two masks 55% Shadow dilution with tube and two masks 50% Ambient illumination 8,000 lux Bulb power consumption 85 W Power supply 100~240 VAC, 50/60 Hz Number of LED bulbs 32 Light head dimension 600 mm Integrated Camera Optional Carrier-arm Camera Optional HyLED 7 Series Contact Us With cutting-edge LED technology, the HyLED 7 Series offers excellent luminous efficiency, heat-free light source, and incredible long service life, giving you total support in OR. Efficient & Economic • Incredible long service time up to 60,000 hours • Central Illumination: 160,000 lux/130,000 lux • Adjustable light field diameter • Max. depth of Illumination : 1,200 mm • Low power consumption Original & Unique • Perfectly integrated into laminar flow (Certified per DIN-1946 Part 4) • Touch screen panel for easier control • 330° rotatable integrated HD/FHD camera • Ultra-thin design & excellent maneuverability • Ergonomic and compact design less than 12kg Ergonomic and Compact Design • The ultra-thin concentrically circular design for better hygiene. • DIN-1946 Part 4 certificated. (12-2008) Flexible Design • Combined with an Ondal spring arm maximizes mobility and ensures stability. • Ultra-thin light head with only less than 12 Kg. • Smooth, metal back cover of the light head. High Performance • With a minimum lifetime of 60,000 hours, each latest generation LED illuminates far longer than traditional light sources. • Easier and less costly maintenance: each LED bulb with individual circuit can be replaced respectively, which will minimize the maintenance cost. Easy and Intuitive Control • Traditional control panel • Touch screen panel(optional) Shadow-free The HyLED 7 Series surgical light inherits the advanced technology from the HyLED 9 series and shows excellent shadow management function. The professional shadow dilution experiment simulated extreme situations by using tubes and masks proved that Mindray HyLED 7 Series surgical light has sufficient illumination even in extreme situations. Camera System for Communication The camera system provides better communication and documenting solutions. The camera system integrated with the surgical lights also provides a platform for medical research purpose, teaching communication and video documenting. Digital video recorder Supporting external USB storage device Supporting HDMI/YPbPr/HD-SDI/DVI-D/VGA input signal Default with camera control function Supporting video recording from other medical devices source
Send Message