Hematology
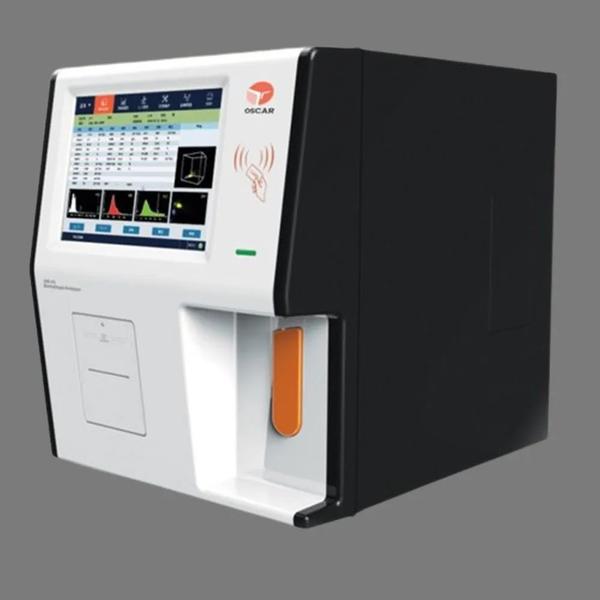
Buy the Best Automated Hematology Analyzers – Acculab CBC 360 Neo. This Hematology Analyser Machine is Truly Automated for self-checking, background test, calibration, sampling, dilution, mixing, printing, dormancy, alarms, cleaning and maintenance. CBC 360 Neo is 3 part hematology analyzer from Accurex & is Automated for Walk-away Operations. Dual Chamber: Measurement of RBC / platelets & WBC / HGB in different chambers ensure accurate counting in a short time. The micro-aperture sizes of both the chambers are well designed to avoid error due to cell misclassification
Automation for Walk-away Operation:
Auto Self-check : Automatic aspiration of reagents & rinsing of tubings on startup
Auto Blank : Background test is run automatically on startup
Auto Print : Automatic printing of report with / without histogram
Auto Sleep : Dormancy status is entered after a set period of non-operation
Auto Alarms : Audio / visual alarms in case of system errors & abnormal results
Auto Clean : Automatic rinsing options available to maintain the system
Dual Chamber:
Measurement of RBC / platelets & WBC / HGB in different chambers ensure accurate counting in a short time.
The micro-aperture sizes of both the chambers are well designed to avoid error due to cell misclassification
Specifications:
Measurement principle: Electrical resistance for counting RBC, WBC & Platelets Colorimetric estimation of HGB at 540 nm
Parameters: WBC, LY, MO, GR, LY%, MO%, GR%, RBC, HGB, HCT, MCV, MCH, MCHC, RDW-CV, RDW-SD, PLT, MPV, PDW, PCT, P-LCR
Throughput: 60 samples/hour
Micro-aperture: WBC : 100 um RBC / PLT : 68 um
Sample type: Whole blood or pre-diluted sample
Sample volume: Whole blood (venous blood) : 10 ul
Chamber: Pre-diluent (capillary blood) : 20 ul, Pre-diluent (capillary blood) : 20 ul
Reagents: Dual chamber : RBC / Platelets & WBC / HGB
Quality control: 4 QC options : L-J, X-B, X-R & X QC, Graphs plotted with 31 data points
Data memory: Upto 100,000 (with histogram)
Display: 10.4 inch LCD with 640 x 480 resolution
Printer: Built-in thermal printer
Interface: Support RS-232C, standard network port and USB; keyboard and mouse
Operating environment: Temperature : 15˚C – 35˚C, Humidity : 85% RH
Electrical specification: Voltage : AC 110V/230V 23V Frequency : 50/60 Hz, Power : 180W Fuse specification : 250V/3A
Dimensions / Weight: 670 x 515 x 640 mm / 27.5 kgs
Specification
Acculab CBC 360 Neo – Fully Automated Hematology Analyzer
Features:
3-part differentiation of WBC
Automated for — self-checking, background test, calibration, sampling, dilution, mixing, printing, dormancy, alarms, cleaning and maintenance
High processing speed : 60 samples/hour
Multi-parameter estimation : 20 parameters categorized according to corpuscle type for easy reporting
Direct command keys for Mode, Prime, Flush, Drain & Record / Print for faster operation
Run / Standby indicator lights denote the analyzer status i.e. test is being run or analyser is ready to test
Whole blood/ Pre-diluent indicator lights denote the sample type in use i.e. whole blood/ pre-diluent
Dual sample mode with low sample volume: Whole blood (venous) mode : 10 ul;
pre-diluent (capillary) mode : 20 ul
Audio alarm indication for abnormal findings or system errors
4 QC methods : L-J, X-B, X-R & X QC
Comprehensive QC graphs with 31 data points for each parameter
Internal & external probe cleaning facility
Dormancy mode available to reduce power consumption
Memory : 100,000 (with histogram)
Safety Information
• Large 10.4 inch single screen color display of all 21 parameters and 3 histograms
• Sequential arrangement of WBC, RBC & PLT parameters for convenient reporing
•Easy to understand & icon-based arrangement of menu for fast and convenient testing
Direction for Use
• WBC Histogram: R1, R2, R3, R4 & RM indications on histogram show specific abnormalities in the histogram & the probable causes
• Platelet Histogram: PM indication is given when the boundary between PLT & RBC is ill-defined thus avoiding misclassification
Other Info
High voltage cautery function is useful in disintegrating obstinate protein or serum clog in the tubing
Send Message