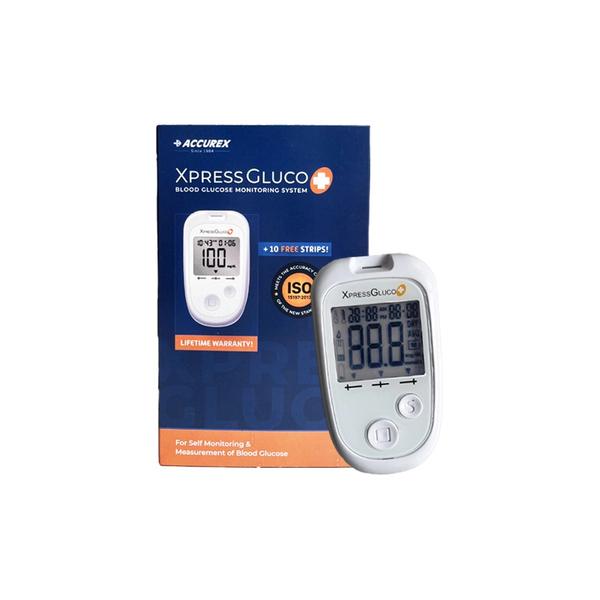
Accu-Chek® Instant Blood glucose monitor Your effortless choice Item Description Wishing there was an easy way to monitor your blood glucose levels? With the new Accu-Chek Instant meter, it's as easy and fast as checking your phone messages. There is no setup required – just insert a test strip, apply a small blood sample and read your blood glucose result on the large display. Additional specifications Model: Accu‑Chek Instant wireless blood glucose meter Meter storage conditions: Temperature: -25 – 70° C Memory capacity: The meter automatically stores at least 720 blood glucose results in memory, but only the last blood glucose result and your 7, 30, and 90 day averages can be viewed on the meter. To view stored blood glucose results, transfer them to a compatible software application. The meter automatically stores at least 30 control results in memory, but only the current control result can be viewed on the meter. To view stored control results, transfer them to a compatible software application. Sample claims: Capillary: Yes Venous: Yes Arterial: Yes Neonate: Yes Non-fingerstick sample Claim: Palm, forearm, upper arm Measuring principle: FAD glucose dehydrogenase (GDH) Measuring time: 4 Sec Measuring conditions: 4 °C to 45 °C (39 °F to 113 °F) Hematocrit range: 10 to 65 % Humidity range: 10 to 90 % Altitude independence: 0 to 3,094 m (10,150 ft) Automatic off: 90 seconds after performing a test, 15 seconds after test strip is removed, or 5 seconds from the last test result screen. Weight: Approx. 40 g (with batteries) Construction: Hand-held Protection class: III Meter type: The Accu-Chek Instant meter is suitable for continuous operation. Interfaces: Connectivity using BLE wireless Technology to mySugr App; USB interface with USB cable to transfer data to the Accu‑Chek Connect Online system. Benefits and Features Effortless Bluetooth synchronization with mySugr diabetes management app The intuitive target range indicator gives you visual reassurance. It can be individualized to suit your personal therapy goals Only what you need: Your test result and your averages (7/30/90 days) are visible on the meter Sleek, modern design with large, easy-to-read BG numbers and a bright, backlit display No set up required – just insert a strip, apply a small blood sample, and read your blood glucose result on the large display Easy-edge test strip: Apply a small drop of blood anywhere along the wide, yellow edge Proven accuracy guaranteed by the makers of the Accu-Chek brand, fulfilling the requirements of the ISO 15197:2013, EN ISO 15197:2015 All data can be uploaded to the mySugr by using Bluetooth Low Energy wireless technology and to Accu-Chek Connect Online portal by using USB Test strips come in a 10, 25, 50 vial so you can choose whatever fits your budget or therapy Enjoy virtually pain-free testing with the Accu-Chek Softclix lancing device
Send Message