Glycohemoglobin Analysis
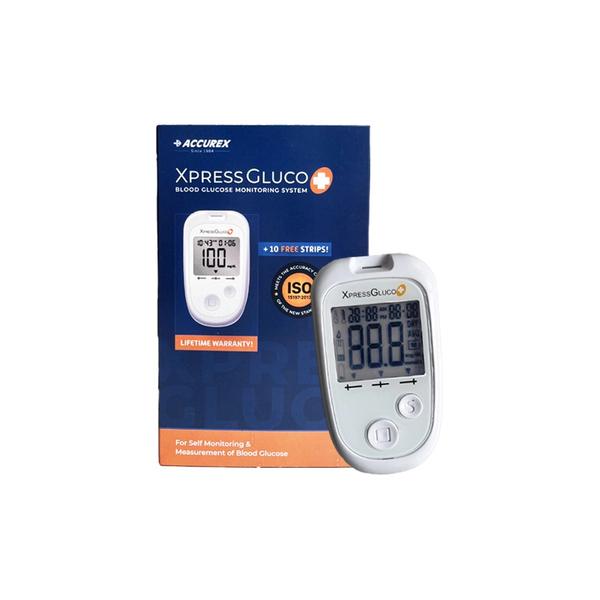
Buy Xpress Glucometer Plus Kit Online, it’s a smart Glucometer Machine kit. This Digital Glucometer consists of 1 Blood Glucose Meter, 10 Test Strips, 1 Multilet Lancing Device, 10 Sterile Lancets, 1 Carry Pouch, 1 Warranty Card, Test Strip Insert Sheet, 1 Battery (3V) Gluco Plus Meter is intended for use in the home & in clinical settings. There are different types of Glucometers in Point of Care (POC). XPRESSGLUCO + meter measures the strength of the electrical current, calculates your blood glucose level and then displays your result in either milligrams of glucose per deciliter (mg/dL) or millimoles of glucose per liter (mmol/L).
The system is intended for use outside the body (in vitro diagnostic use only). It should be used only for self-testing blood glucose (blood sugar) and only with fresh capillary whole blood samples drawn from the fingertips, forearm, upper arm, palm, calf or thigh. The system is intended for use in the home and in clinical settings. The system should not be used for the diagnosis of diabetes or for the testing of newborns.
Blood glucose is measured by an electrical current that is produced when a blood samples mixes with the reagent (special chemicals) of the test strip. The electrical current changes with the amount of glucose in the blood sample. The XPRESSGLUCO+
meter measures the strength of the electrical current, calculates your blood glucose level and then displays your result in either milligrams of glucose per deciliter (mg/dL) or millimoles of glucose per liter (mmol/L).
Safety Information
1. The user should not take any decision of medical relevance without first consulting his or her medical practitioner.
2. Call your doctor immediately if you experience symptoms that are not consistent with your blood glucose test results.
3. High altitudes above than 3,402 meter (11,161 ft) may affect the test results.
4. Temperatures outside the range of 10°C to 40°C (50°F to 104°F) may affect the test results. Do not test beyond of temperature range.
5. Do not perform servicing and maintenance while the meter is in use.
6. No modification of this equipment is allowed.
7. Do not use this meter near cellular or cordless telephones in a call, walkie-talkies, garage door openers, radio transmitters, or other electrical or electronic equipment that are sources of electromagnetic, radiation, as these may interfere with the proper operation of the meter.
Direction for Use
1. Wash you hands and puncture sit: Wash your hands in warm, soapy water. Rinse and dry completely . Warm your fingers to increase blood flow.
2. Insert Test Strip : Remove a new test strip from vial. Be sure to tightly replace vial cap after removing test strips.
Insert a test strip with the contact bar end entering into the test strip slot first. Push the test strip as far as it will
go without bending it. The meter turns on automatically.
3. Hold the prepared lancing device firmly against the side of your fingertip. Press the release button. (NOTE : If you want to do alternative site testing, please refer to the “About Alternative Site Testing (AST)” section. Please consult your healthcare professional before obtaining blood from site other than your fingertip.)
4. Obtain a Blood Sample : Gently massage your finger or puncture site to obtain the required blood volume. To
perform the test, you need only 0.5 μL of blood sample. Do not smear the blood sample. To obtain best accurate result,
wipe off the first drop of blood and gently squeeze another drop of blood.
5. Apply Blood Sample : When the meter shows the “ ” symbol, apply blood to the opening of the absorbent channel of the
test strip where it meets the narrow channel. Blood will be drawn into the test strip. If the test strip confirmation window is full, you will hear a beep.
6. Read Your Result : After the meter counts down from 5 to 1, your blood glucose test result appears along with the unit of measure, date and time. Test result is lower than the target range Test result is within the target range. Test result is higher than the target range *The default target range is 70 mg/dl to 180 mg/dl.
7. Turn Off the Meter : This blood glucose result is automatically stored in the meter memory. Turn the meter off by removing the test strip. Discard the used test strip carefully to avoid contamination.
8. After use, twist off the Lancing Device Cap. Push the exposed tip of the lancet into its Protective Cap.
9. Slide the Lancet Ejector forward and disposing the used lancet in an approved container. Discard the used lancet according to your country’s safety regulations. Replace the Lancing Device Cap.
Other Info
•Use only XPRESSGLUCO+ Control Solution with your XPRESSGLUCO+ meter.
•Check the expiration date on the bottle. Do not use if expired.
•Use within a period of 90 days from the date that you first open it. Record the discard date on the control solution bottle when you first open it to serve as a reminder to discard after 90 days.
•The control solution ranges are printed on the label of the XPRESSGLUCO+ Blood Glucose Test Strip vial. They are not recommended target ranges for your blood glucose.
•For in vitro diagnostic use.
•Do not add any liquid to the XPRESSGLUCO+ Control Solution.
•Do not take internally or inject.
Send Message