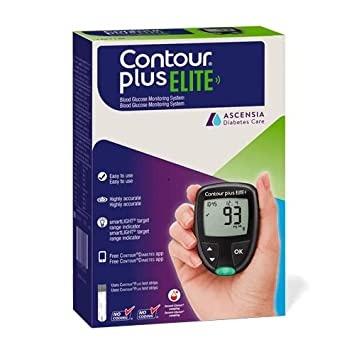
ONTOUR®PLUS ELITE blood glucose monitoring system. Discover high accuracy1 and ease of use - the features you want from a meter. Product Features High accuracy1 smartLIGHT® target range indicator Second-Chance® Sampling - 60 seconds Easy to use Connects to the free CONTOUR®DIABETES app** An Easy-To-Use System The features you want from a meter2. Easy to read: Big numbers on display. Easy to use: Large rubber buttons. No initial set up.* Personalized target ranges with averages on the meter. Discover smartLIGHT® for easier understanding of blood glucose levels3 The unique smartLIGHT® feature makes it quicker and easier to interpret blood glucose readings using coloured lights that clearly identify if the reading is above, within or below your target range.† Discover Second-Chance® sampling which may help save test strips4 CONTOUR®PLUS ELITE has a 60 second count down screen that provides patients the opportunity to apply more blood to the same strip if the first sample is insufficient. onnects to the free CONTOUR®DIABETES app The features you want from a meter2. Easy to read: Big numbers on display. Easy to use: Large rubber buttons. No initial set up.* Personalized target ranges with averages on the meter. The free CONTOUR DIABETES app is available to support diabetes self-management, adding insight and meaning to the results Easy to use: Syncs automatically with the meter to upload all blood glucose readings to an electronic diary Easy to understand: My Patterns helps identify trends in blood glucose results and displays notifications of potential causes Easy to share: The blood sugar diary report can be sent prior to, or shared during, an appointment with a healthcare professional The CONTOUR®DIABETES app applies appropriate safeguards to ensure your personal data is processed securely and in compliance with applicable laws.
Send Message