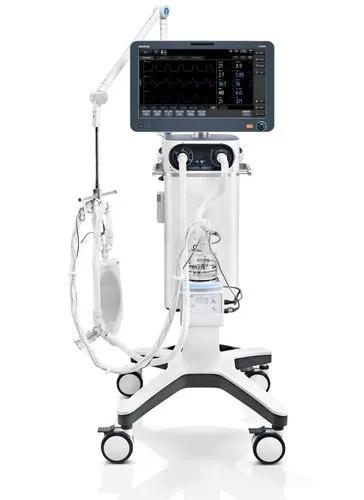
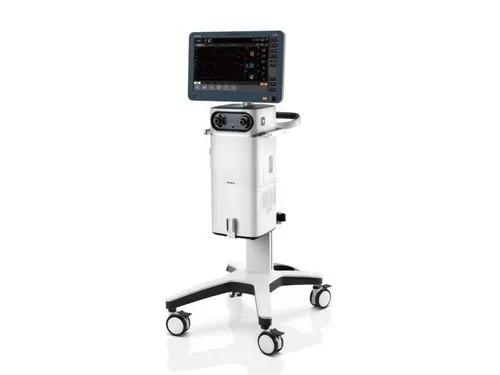
Operate with Ease In the modern busy clinical environment, ease of use is a fundamental requirement for all medical devices. With this in mind, the new SV600 ventilators enable clinicians to set and deliver ventilation therapies quickly and easily via the intelligent ergonomic design and flat user interface. PulmoSight TM Utilizes numerical and graphical displays to show real time resistance, compliance and the spontaneous breathing status. Combined with the dynamic short trend display clinicians are able to monitor and evaluate changes in the patient's pulmonary ventilation and initiate the appropriate therapies. User configurable UI The SV600 ventilators offer exceptional user flexibility. Users are able to configure frequently used parameter controls by making them quick access shortcut keys in the UI. Also the ventilation mode keys can be arranged in order of frequency of use. This enables you to customize device in your way making parameter adjustment easier and quicker. Graphic guidelines The new intuitive graphical display enables users to learn quickly how to navigate and locate mode and parameter controls, thereby reducing errors and improving efficiency. Minimal Maintenance Routine maintenance requires no tools. The new 'door design' means that no tools are required to perform regular routine maintenance of the oxygen sensors, water trap, fan dust filter, HEPA air intake dust filter, etc. This ensures your new device always remains clean and clutter free. Intelligent Decision Making Ventilation modes and decision-supporting tools like Intelligent Assistant are developed on the basis of clinical needs and professional guidelines to help medical personnel calmly make clinical decisions. An extensive range of ventilation modes Smart ventilation solution: AMV TM + IntelliCycle TM Emergency solution: CPRVTM Sequential treatment regimen: Non-invasive ventilation & high flow oxygen therapy Dual channel auxiliary pressure measurements Lung protection kit Expandable Capabilities Securing your devices future is reliant on being able to expand your devices capabilities by interacting or integrating new concepts and technologies. The new SV800 / SV600 ventilators allow just this. Continue the latest electronic software and hardware, your new device is ready to embrace new technological advancement with ease. Integrated neonatal module with tidal volumes as low as 2 ml Plug & Play modules , including SpO2, mainstream or sidestream CO2 Backup air supply in case of air supply failure Seamlessly connected to the hospitals clinical information system via the Mindray BeneLink solution or eGateway solution Brand Mindray Model Name/Number SV600 Patient Age Group Neonatal, Adult, Paediatric Mounting Type ICU Operating Type Automatic Display Full color 15.6 inch Resolution 1920x1080 pixel Compliance IEC 60601-1-2 for EMC
Send Message