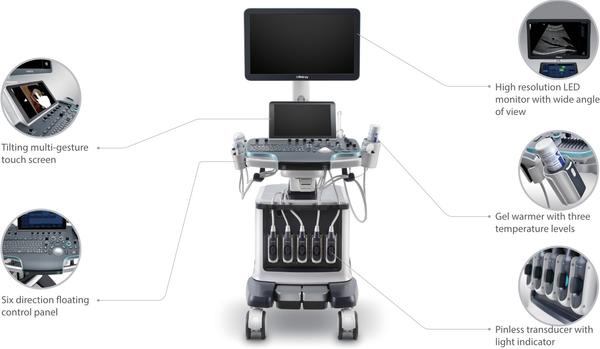
Since the company was founded, Mindray has been continuously exploring new ways to improve diagnostic confidence. Powered by the most revolutionary ZONE Sonography® Technology, Resona 7’s new ZST+ platform brings ultrasound image quality to a higher level by zone acquisition and channel data processing. As well as the premium level image quality, Resona 7 also enhances clinical research capabilities with the revolutionary V Flow for vascular hemodynamic evaluation, and the most intelligent plane acquisition from 3D datasets for fetal CNS diagnosis. Combining the most intuitive gesture-based multi-touch operation and all the essential clinical features, Resona 7 is truly leading new waves in ultrasound innovation. With core platform advantages of ZST+ The channel data based ZST+ is an extraordinary innovation, representing an ultrasound evolution. Transforming ultrasound metrics from conventional beamforming to channel data based processing, ZST+ is able to deliver multiple imaging advances: Advanced Acoustic Acquisition, Dynamic Pixel Focusing, Sound Speed Compensation, Enhanced Channel Data Processing and Total Recall Imaging. Advanced features V Flow V Flow (Vector Flow) is a novel approach for vascular hemodynamic analysis. V Flow uses color coded vector arrows to indicate the velocity’s magnitude and direction of blood cells. With ultra-high frame rates, it provides extremely accurate and angle-independent visualization of complex vascular hemodynamic profiles. With comprehensive data information, V Flow is the most valuable tool for vascular clinical research. resona-7-fig3-1-pc UWN+ Contrast Imaging UWN+ (Ultra-Wideband Non-linear Plus) CEUS enables the Resona 7 to detect and utilize both the 2nd harmonic and non-linear fundamental signals, generating significantly enhanced images, resulting in greater sensitivity of minor signals and longer agent duration with lower MI. Forwarding smart to clinical intelligence The Resona 7 elevates clinical intelligence with a complete solution that enables clinicians to manage both routine and advanced studies more efficiently,consistently, and accurately, from acquisition to calculation. As an example, Smart Planes CNS shows exceptional intelligence in accurate diagnosis and analysis of the fetal central nervous system (CNS) Smart Planes CNS Mindray’s exclusive pioneering technology positions the Resona 7 as the industry’s first ultrasound system to allow fully automatic and accurate detection of the most significant planes and frequently used measurements of fetal CNS, leading to intelligent diagnosis, improved throughput, and reduced user dependence. Smart Planes CNS provides a user-friendly tool that greatly improves scanning efficiency through increased accuracy coupled with automated operation. With a simple button click on a 3D fetal brain volume image, the standard CNS scanning planes (MSP, TCP, TTP and TVP) and a range of related anatomical measurements (BPD, HC, OFD, TCD, CM and LVW) are obtained immediately. Smart Face Acquiring an optimal view of the fetal face in 3D ultrasound is cumbersome and time-consuming. In some cases, it is impossible to get rid of the occlusions such as cord, placenta, uterus and extremities. The new Resona 7 with Zone Intelligence provides a fast and intelligent optimization for fetal face with simply one-touch. It can immediately remove occlusions in the volume data, eliminate unwanted noise information, and generate an optimal view of the fetal face with minimal effort. Ergonomics The Resona 7 is designed around you. Gesture-based operation opens up a new trend in cart-based ultrasound with an agile, smart, and intuitive user experience beyond your expectations. Gel warmer’s three level temperature and swiveling angle adjustment of the control panel delivers great patient comfort and user convenience.
Send Message