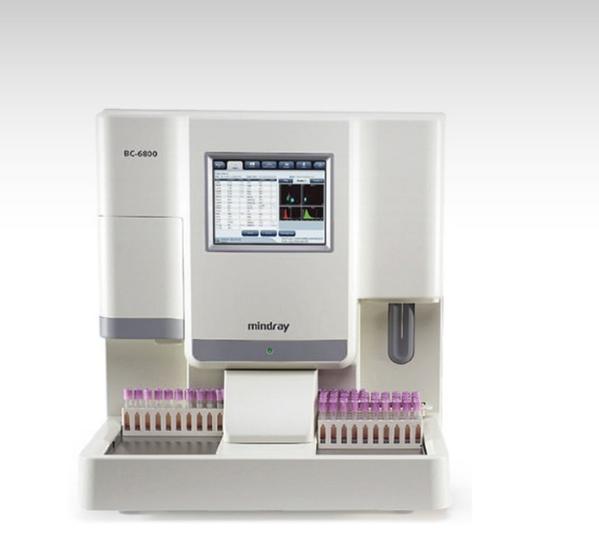
BC-6800 Today, laboratories not only need more reliable routine CBC plus 5-part diff WBC testing by way of high processing speed, but are also looking for options that extend the analyzer's output in form of Reticulocytes, Nucleated RBCs, Fluorescent Platelet counts etc. for wider clinical application and research. On the other hand, they are restricted by limited budgets. BC-6800 can help to meet all these needs just perfectly and even exceed the expectations. Additional parameters for further clinical applications IMG(#,%) parameters provide information about immature granulocyte, including promyelocytes, myelocytes and metamyelocytes. HFC*(#,%) parameters represent high fluorescent cell population, such as blasts and atypical lymphocytes. RET(#,%) extend valuable help in the differential diagnosis and/or therapeutic monitoring of anemias. IRF is parameter concerning immature reticulocytes, which can assist in early diagnosis of anemia and monitoring the bone marrow response to therapy. InR*(#,‰) are parameters regarding infected red blood cell. The red blood cells may infected by plasmodium, which can cause malaria. * means research parameters SF Cube Technology SF Cube is a pathbreaking technology for reliable blood cell analysis, including WBC differential, Reticulocytes and NRBC with efficient flagging. After reaction with proprietory reagents, the targeted blood cells undergo 3D analysis using information from scatter of laser light at two angles and fluorescence signals. The 3D scattergram builds the power to better identify and differentiate blood cell populations, especially to reveal abnormal cell population undetected by other techniques. Minimizing interference to ensure more accurate results NRBCs are counted in a dedicated channel by SF cube method, which automatically corrects total WBC count and 5-part Diff results when NRBCs are detected. The Focusing Flow-DC method minimizes the interference traditionally encountered in DC technology to produce near gaussian histograms. PLT-O result avoids the interference from microcytic and fragmented RBCs, large platelets and/or platelet clumps by fluorescent stain, and enhances the results accuracy and sensitivity. High automation to minimize the workload Throughput for 125 tests/hour, autoloader for 100 sample tubes capacity Bi-directional LIS with HL7 or ASTM communication protocol Customizable re-exam rules allow users to define their re-exam criterion according to lab’s practice
Send Message